Tiziana Life Sciences PLC (TLSA: NASDAQ) Clinical Improvements for 2nd SPMS Patient
Research Note
Clinical Results for Second SPMS Patient
Patient Response
The second patient was diagnosed with SPMS in 2014 and over the subsequent eight years, the magnitude of his disability increased. After enrolling, the patient was administered three months of treatment with intranasal foralumab at 50 mcg, three times per week for two weeks, followed by one week off of treatment. Improvement was measured by PET imaging and by neurologic examination. A 10-30% reduction in microglial activation was observed in the PET imaging across the thalamus, cortex, white matter and cerebellum, which is similar to the ranges observed in the first patient. See link here for the discussion on the results for the first patient and detailed background on SPMS. In the clinical sphere, investigators observed improvements in the 25-foot walk test and the neurologic exam for patient #2. Both enrollees are continuing their treatment regimen with foralumab and are now in their 13th and 4th months of treatment.
Next Steps
The positive data from the first two patients was shared with the FDA, which granted permission for Tiziana to add eight more SPMS patients to the trial. Reassuring safety results supported the option to use higher levels of dosing, and for all patients going forward, doses may be escalated to up to 100 mcg three times per week1 to investigate the potential clinical benefits from higher doses. Tiziana expects the third patient to be enrolled in the trial in
While only two patients have contributed to the data so far, the positive response is promising and supports further investigation for this condition that affects near 300,000 individuals around the globe. While there are a few treatments for the specific disorder, the agents act by modulating or suppressing the peripheral immune response and have limited effect on progressive forms of multiple sclerosis.
1 The regimen is a three times per week for two weeks followed by one week off of treatment.
SPMS Background
Tiziana enrolled its first SPMS subject in
SPMS represents an advanced stage of multiple sclerosis with few treatment options and has a severe impact on a patient. Three-month results released in January of this year provided sufficient evidence of safety and efficacy to justify the enrollment of a second SPMS subject. On
Intranasal Foralumab’s First SPMS Patient: 3- and 6-Month Data, KOL Discussion
On
As disclosed in the
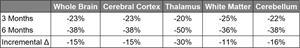
Exhibit I - 6-Month Reduction in Microglial PET Signal from First SPMS Patient3
Proposed Phase Ib Foralumab Trial: Crohn’s Disease
Tiziana expects to launch a double-blind, multiple ascending dose study of orally administered foralumab to assess the safety of foralumab in patients with mild to moderate Crohn’s Disease, with the first patient to be enrolled in late June. 2.5 mg and 5 mg doses in take-home capsules will be administered for five consecutive days and will be evaluated for safety and tolerability. Sixteen subjects are expected to be enrolled with two dose groups and a 3:1 active to placebo ratio in each group. During the five day hospitalization period for the subjects enrolled, vital signs will be evaluated, laboratory values will be recorded and adverse events and other physical findings will be measured to assess the safety and tolerability of the agent. Secondary endpoints include measurement of systemic circulation of foralumab and pharmacokinetics, change from baseline in inflammation response and incidence of detectable anti-drug antibody. Exploratory endpoints will include change in T-cell subsets in blood from baseline and detection of foralumab in stool samples.
2 SUVR-1 is calculated with reference to a pseudo reference region in white matter that showed minimal change in PET SUV across time points
3
Annual Report
Tiziana filed its annual report, Form 20-F, for the fiscal year ended
The
Exhibit II – Tiziana Pipeline4
Milestones
- First Crohn’s patient enrolled –
June 2022 - Submit IND for foralumab in Type 1 Diabetes –
July 2022 - Submit IND for Milciclib in non-small cell lung cancer (NSCLC) –
July 2022 - Enroll third patient in SPMS trial –
July 2022 - Submit IND for Anti IL-6 (TZLS-501) for pulmonary fibrosis – Fall 2022
- Topline data from SPMS trial - 2023
Summary
Tiziana has shown early success with its first two patients in the SPMS trial with clinical benefit observed after three months in the second patient. Additional SPMS subjects will be added as the year progresses and we expect to see topline data from the study next year. In other programs, we expect to see an IND submission for foralumab in Type 1 Diabetes, for Milciclib in NSCLC and for TZLS-501 in pulmonary fibrosis later this year.
4 Source:
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service
For further inquiries:
and Investor Relations Manager
+44 (0) 207 495 2379
email: info@tizianalifesciences.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/ec0d68fb-4ed5-4a96-b181-aa096959f477
https://www.globenewswire.com/NewsRoom/AttachmentNg/bd539bfe-0373-423e-872a-07ae151c3c58

Source: Tiziana Life Sciences Ltd.