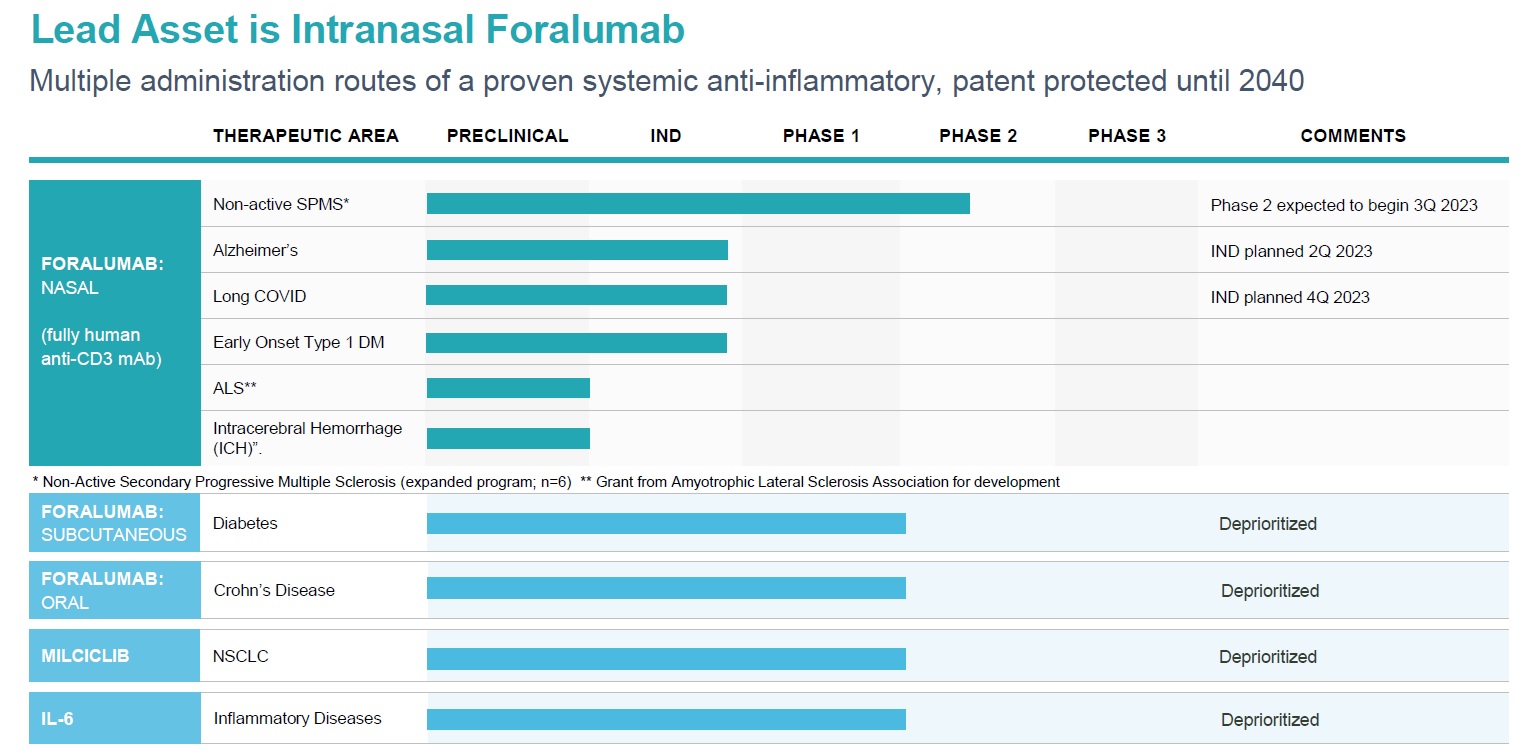
TLSA Pipeline
Foralumab (TZLS-401)
Foralumab is a fully human anti-CD3 monoclonal antibody (mAb) for the treatment of Crohn’ s and neurodegenerative diseases. We have completed two Phase 1 clinical trials: one for progressive MS indication with nasal administration and the other for Crohn’s disease indication, with enteric coated capsules administered orally. We also completed a Phase 2 trial treating mild to moderate non-hospitalized COVID-19 patients in Brazil with intranasal foralumab with positive results (Moreira et al., 2021)*. Currently, two secondary progressive MS patients are being treated at Brigham and Women’s Hospital, Boston MA, with intranasal foralumab under Expanded Access INDs with signs of clinical benefit, we are expecting 12-month data from EA#1 and 6-month data from EA#2 in 4Q-2022. Patient enrolment for the Intermediate-size patient population expanded program has begun. Foralumab has demonstrated ability to activate regulatory T cells that systemically circulate to elicit targeted immunomodulation providing therapeutic benefit to patients.
Tiziana has recently submitted a patent application on potential use of Foralumab, to improve success of chimeric antigen receptor T cells (CAR-T) therapy for cancer and other human diseases. The patent application covers inventions related to improving CAR-T expansion and/or survival. Foralumab administered alone or co-administered in combination with co-stimulatory molecules, such as an anti-IL-6 receptor monoclonal antibody, an anti-CD28 monoclonal antibody or specific inhibitors of signalling pathways of phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT), or mammalian target to improve success of CAR-T therapy.
* Moreira, T. G., et al. (2021) Nasal Administration of Anti-CD3 Monoclonal Antibody (Foralumab) Reduces Lung Inflammation and Blood Inflammatory Biomarkers in Mild to Moderate COVID-19 Patients: A Pilot Study. Front Immunol 12, 709861
Milciclib (TZLS-201)
Milciclib is a potent, small molecule inhibitor of multiple cyclin-dependent kinases (CDKs), tropomycinreceptor kinases and Src family kinases controlling cell growth and malignant progression of cancer. Milciclib has demonstrated safety and tolerability in 316 patients with advanced solid cancers in Phase 1 and 2 studies and also exhibited positive clinical responses. In two, successfully completed, Phase 2 thymic cancer trials, Milciclib successfully increased overall survival and met both primary and secondary endpoints.
In July and September 2019, we reported positive Phase 2a safety, tolerability and efficacy data of Milciclib as a monotherapy in 28 patients with advanced HCC. The results, presented at ASCO2020, warrants further clinical development. Strong genetic and pharmacological evidence suggests that pan-CDKs inhibitors might have potential to suppress the multiple tumorigenic pathways that are activated due to activation of KRAS gene. Clinical data from a Phase I dose-escalation study with combination of milciclib with gemcitabine showed significant disease stabilization and suggested that milciclib can reverse gemcitabine-resistance in NSCLC refractory solid tumors. The clinical response in the NSCLC patient was particularly very promising. Company is exploring the combination of milciclib and gemcitabine in NSCLC subjects with pan KRAS-positive mutations.
TZLS-501
Tiziana's Anti IL-6R mAb (TZLS-501), a fully human mAb binds to both membrane-bound and soluble forms of IL-6R, an inflammatory cytokine driving chronic inflammation associated with autoimmune disease and cancer, reducing circulating levels of the IL-6 cytokine. Anti-IL-6R antibody can potentially be used in combination with Foralumab or other anti-inflammatory and anti-infective agents as therapy for idiopathic pulmonary fibrosis (IPF), acute respiratory distress syndrome (ARDS), multiple myeloma, arthritis, lupus and oncology indications. Excessive production of IL-6 is regarded as a key driver of chronic inflammation and is believed to be associated with severe lung damage and chronic fibrosis observed with acute and chronic respiratory illness.
The Company is scaling GMP manufacturing of its anti-IL-6R mAb concurrently with developing a hand-held nebulizer technology for direct delivery of the antibody into the for treatment of patients with IPF, a rare disease indication.